
RESEARCH
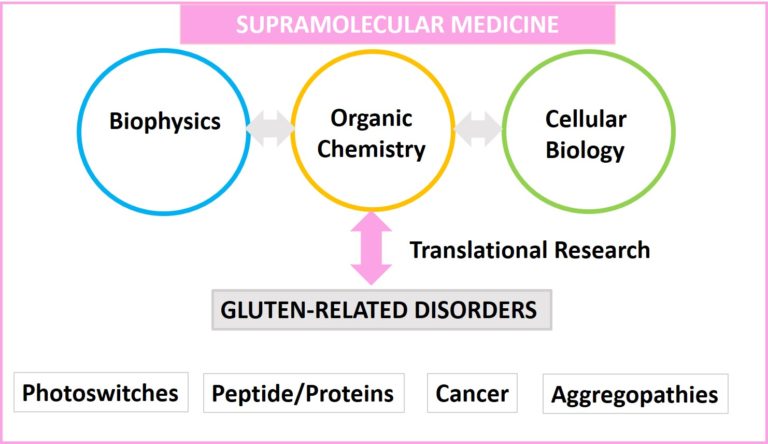
Our research focuses on the design, synthesis, and evaluation of supramolecular systems based on small molecules and peptides that operate under aqueous conditions. We employ different tools from organic chemistry, biophysics, and cell biology at different levels of complexity.
The final aim is to obtain a profound understanding of the relationship between molecular structure, morphology, and function in a biologically relevant environment.
Nowadays, our principal research is directed towards understanding the mechanism of peptide/protein self-assembly and its roles on the delicate balance between health and disease in gluten-related disorders. From our investigations, it is clear that gluten peptides are not only pathogenic for celiac patients. They possess interesting molecular and supramolecular features that could be used to disclose the mechanism of cellular uptake and be translated into other pathologies like aggregopathies and even cancer. This translational research is complemented with other more basic projects in the area of protein/peptide-protein interaction, and functional molecules like photoswitchable biosystems.
Gluten related-disorders and Protein-Protein Interaction

Gluten-related disorders are a group of diseases that involve the activation of the immune system triggered by the ingestion of gluten. These disorders have a high prevalence in Western societies around 5% worldwide because gluten is present in wheat, rye, barley, and some varieties of oats.
It is accepted that the incomplete proteolysis of the gluten proteins is responsible for disease in susceptible individuals. From the chemical perspective, up to now, the research efforts focused on the identification, quantification, and separation of the components of gluten and connecting them with their pathological role in vitro and in vivo. Interestingly, while the primary structure of the molecules involved in the disease is known, there is a lack of information about their intrinsic behavior –such as their folding and molecular organization- under physiologically relevant conditions.
Gliadin and its 33-mer fragment have a central pathogenic role in the context of gluten-related. The aim of the present project is to elucidate the molecular, structural, and supramolecular basis of 33-mer oligomerization and the role of the 33-mer nanostructures in the delicate balance between health and disease.
Oligomerization and conformational transition, towards the β- parallel structure, are hallmarks of Alzheimer’s disease, Parkinson’s disease, and prion disease.
Based on these similarities, the relationship between 33-mer accumulations, due to proteolytical resistance, followed by oligomerization can be the up to now unknown trigger of disease before the onset of inflammation. This hypothesis opens new avenues to understand and treat this common pathology.
To prove this hypothesis, we moved from chemical and biophysics to immunological research, reporting for the first time that only large structures of 33-mer induce an innate immune response in macrophages which is mediated by Toll-like receptor (TLR) 4 activation. This result opens the understanding of the early stages of the disease. It connects activation of the innate immune system triggered by the presence of 33-mer protofilaments for the first time.
The current project combines different chemical and biological methodologies and approaches by including peptide chemistry, structural and supramolecular characterization methods, cellular biology approaches, proteomics, and super-resolution optical microscopy. By combining my research expertise and those of my collaborators, it will be possible to quantify and understand the role of the 33-mer oligomers as modulators of disease.
Our findings will open new avenues in the understanding of gluten-related disorders directed towards the design of new therapeutic targets beyond the gluten-free diet.
Funding: Deutsche Forschungsgemeinschaft (DFG)
Examples of Functional Molecules
Remote Optocontrol of Biomembranes
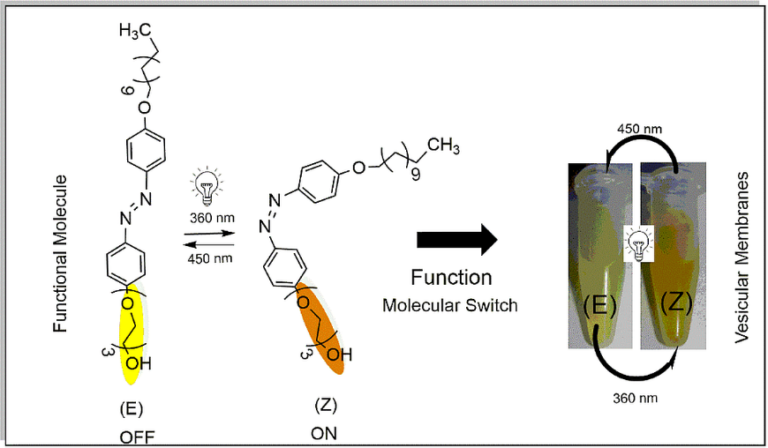
L. A. Benedini, M. A. Sequeira, M. L. Fanani, B. Maggio, V. I. Dodero, J. Phys. Chem. B 120 (2016) 4053
Self-delivering Systems
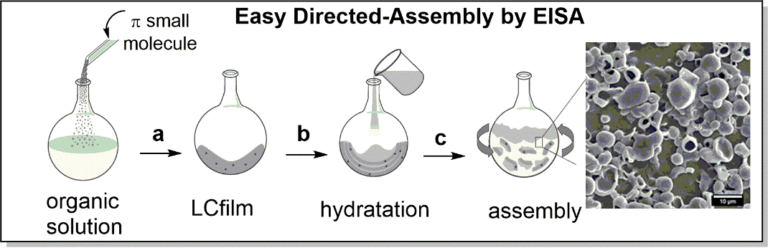
Schematic EISA protocol. Adapted from M. A. Sequeira, M. G. Herrera, Z. B. Quirolo, V. I. Dodero, RSC Adv. 6 (2016) 108132
Controlling Amyloid Formation
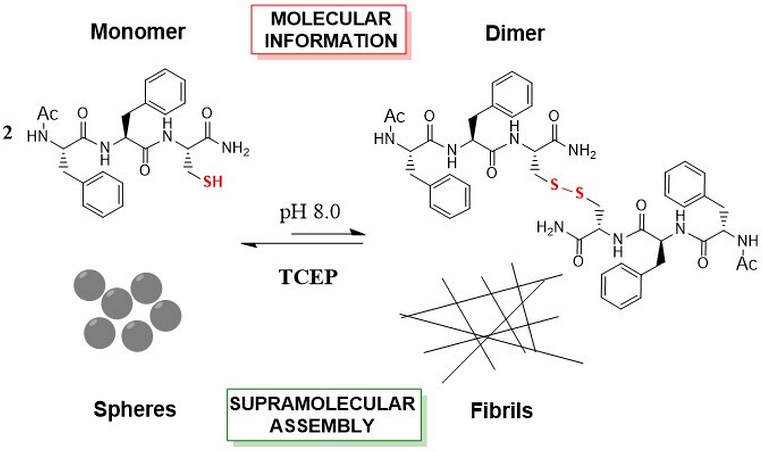
The FFC supramolecular assembly depends on the encoded molecular information which is controlled by the oxide-reduction environment. From M. A. Sequeira, M. G. Herrera, V. I. Dodero Phys. Chem. Phys. , 2019, 21, 11916-11923.

Veronica Dodero
2003 Doctoral Thesis in Organic Chemistry. Supervised by Prof. J. C. Podesta (Universidad del Sur, Argentina) and Prof. T. Mitchell (University of Dortmund, Germany)
1998 Licenciado in Chemistry (BSc. Major). Universidad del Sur, Argentina
Since 2019 Principal Investigator, Faculty of Chemistry, OCIII, University of Bielefeld, Germany
2018 Guest Researcher in Prof N. Sewald’s group, University of Bielefeld, Germany
2015-2018 Georg Forster Fellow, Alexander von Humboldt Foundation (AvH)-HERMES, Faculty of Chemistry, Bielefeld University, Germany
2009-2015 Adjunct Professor of Organic Chemistry, Department of Chemistry, Universidad del Sur (UNS), Argentina
2008-2015 Adjunct Researcher, Organic Chemistry and Biomolecular Interactions, CONICET Argentina
2007-2007 Postdoctoral Researcher in Medicinal Chemistry with M. L. López Rodríguez, Complutense University of Madrid, Spain
2003-2007 Postdoctoral Researcher in Chemical Biology with J.L Mascareñas, University of Santiago de Compostela, Spain
2001-2002 Staff Assistant, Dortmund University, Germany
1998-2009 Staff Assistant in Organic Chemistry, UNS, Argentina
2018 Board Member Marie Curie Alumni Association German Chapter
2018 Visiting Professor, University of Seville, Faculty of Pharmacy, Spain
2015 Georg Forster Fellowship for Experienced Scientists-HERMES, Alexander von Humboldt Foundation
2010 DAAD Visiting Researcher, Bielefeld University, Germany.
2004 Fellowship of the Galician Government, University of Santiago de Compostela, Spain.
2004 Aaron and Fanny Fidelef de Nijamkim’s Award, UNS Best PhD student in Chemistry Year 2003, Argentina.
2000 DAAD fellowship, University of Dortmund, Germany
1998 CONICET fellowship, Argentina.
1998 Intercampus fellowship, University of Girona, Spain
1996 Universidad del Sur Research Student fellowship, Argentina
Since 2023 Member of Global Health Hub Germany - Community on Global Health and Migration
Policy Brief
54+
Publications
950K
Finished Projects
2+
Awards Winning
News
Stay tuned and updated by the latest updates from our blog.

Get in touch
We're always on the lookout to work with new ideas. If you're interested in working with us, please get in touch in one of the following ways.


